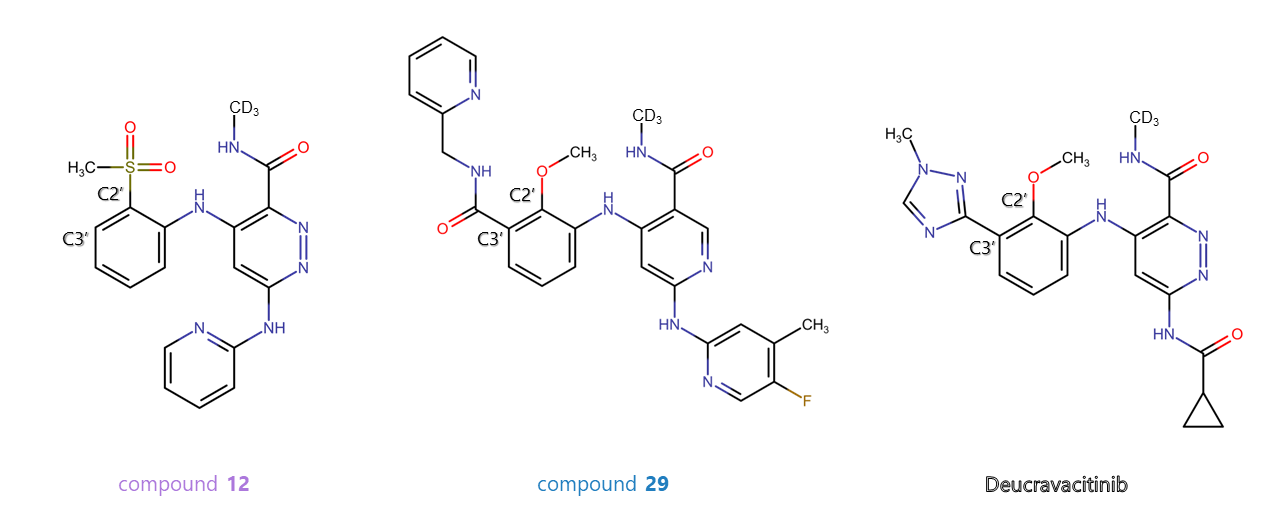
TYK2
January 2023
Tyrosine kinase 2 (TYK2)
Tyrosine kinase 2 (TYK2) is a non-receptor tyrosine kinase belonging to the Janus family (JAK). It functions as a mediator in signaling for several cytokines which makes it an attractive therapeutical target for a variety of inflammatory and autoimmune diseases. Most of the so-far developed small molecule inhibitors target the ATP-binding site of the catalytically active domain of the TYK2 called JH1. However, TYK2-JH1 is structurally very similar for all the members of the JAK family, leading to poor selectivity of these drugs, which show dose-limiting side effects.
More recently, selective, allosteric inhibitors have been developed and are showing very promising results. They target a different region of TYK2, the regulatory domain JH2, which shows higher diversity among kinases of the JAK family and others.
In September 2022, the FDA approved the first-in-class TYK2-JH2 inhibitor from Bristol Myers Squibb (deucravacitinib) for the treatment of moderate-to-severe plaque psoriasis, while in December of the same year, Takeda acquired a best-in-class TYK2-JH2 inhibitor developed by Nimbus Therapeutics, which successfully completed a Phase 2b clinical study.
Here we focus on the development of BMS’s Deucravacitinib, describing its structure-guided design approach and the “water displacement optimization strategy” that produced the drug candidate.
An initial hit-to-lead effort led the scientists to the preclinical, proof-of-concept compound 12, which showed excellent selectivity but needed an improvement in potency. From the 3D structure of its complex with the TYK2-JH2 domain, they gained structural insights that drove the optimization.
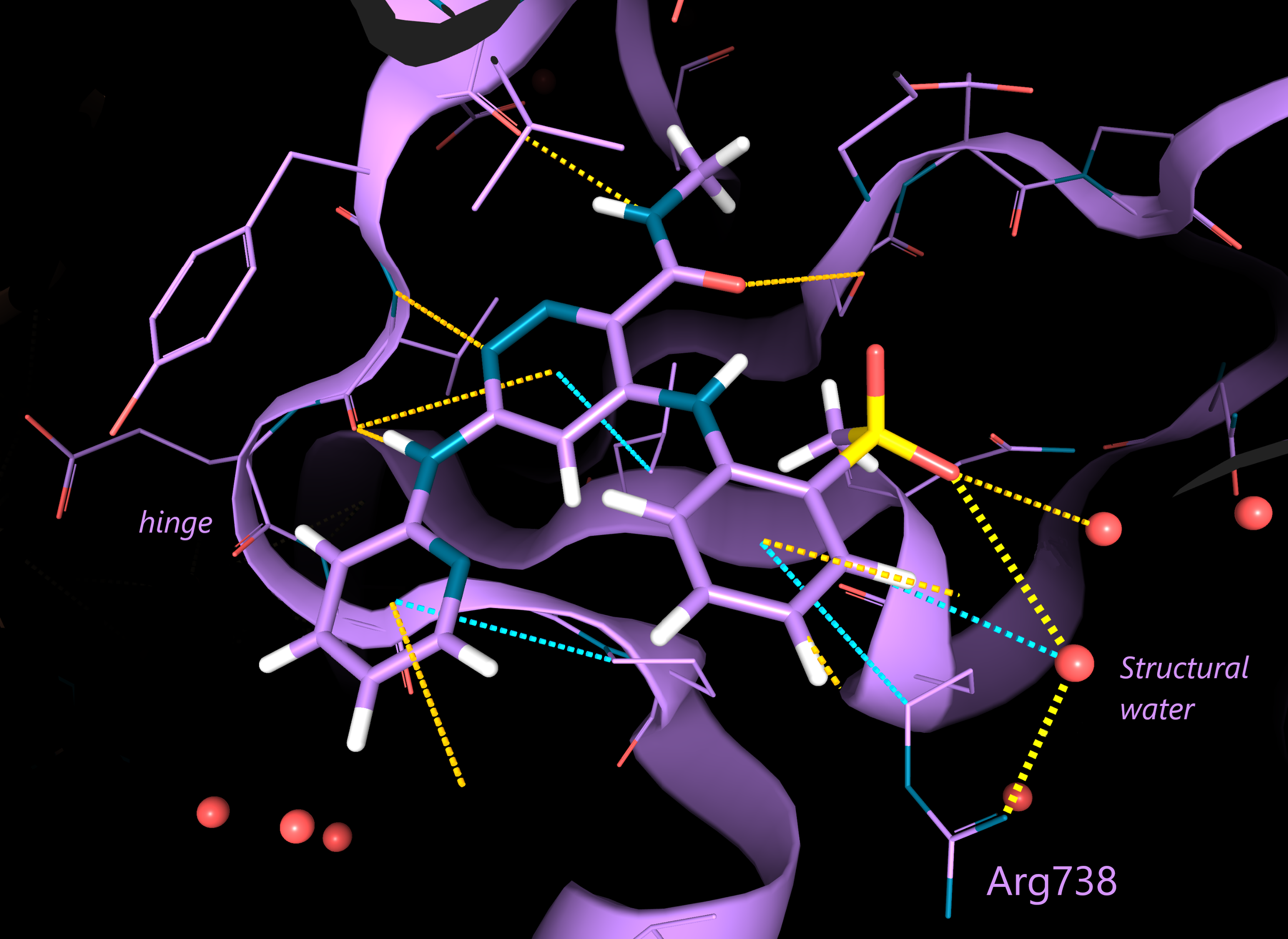
The scientists observed that one of the oxygens of the compound 12 sulfone binds to a water molecule, that forms hydrogen bonds with Arg738. From this observation, they decided to focus the ligand optimization on this portion of the molecule, with the aim of displacing the observed structural water.
Image 1. 3D structure of compound 12 in complex with TYK2-JH2 (purple; PDB: 6NZR). Astructural water mediates the interaction with the residue Arg738. The protein hinge region is indicated for reference. Electrostatic interactions in yellow, aromatic ones in blue (interactions automatically calculated by 3decision® software). Picture produced with the 3decision® software.
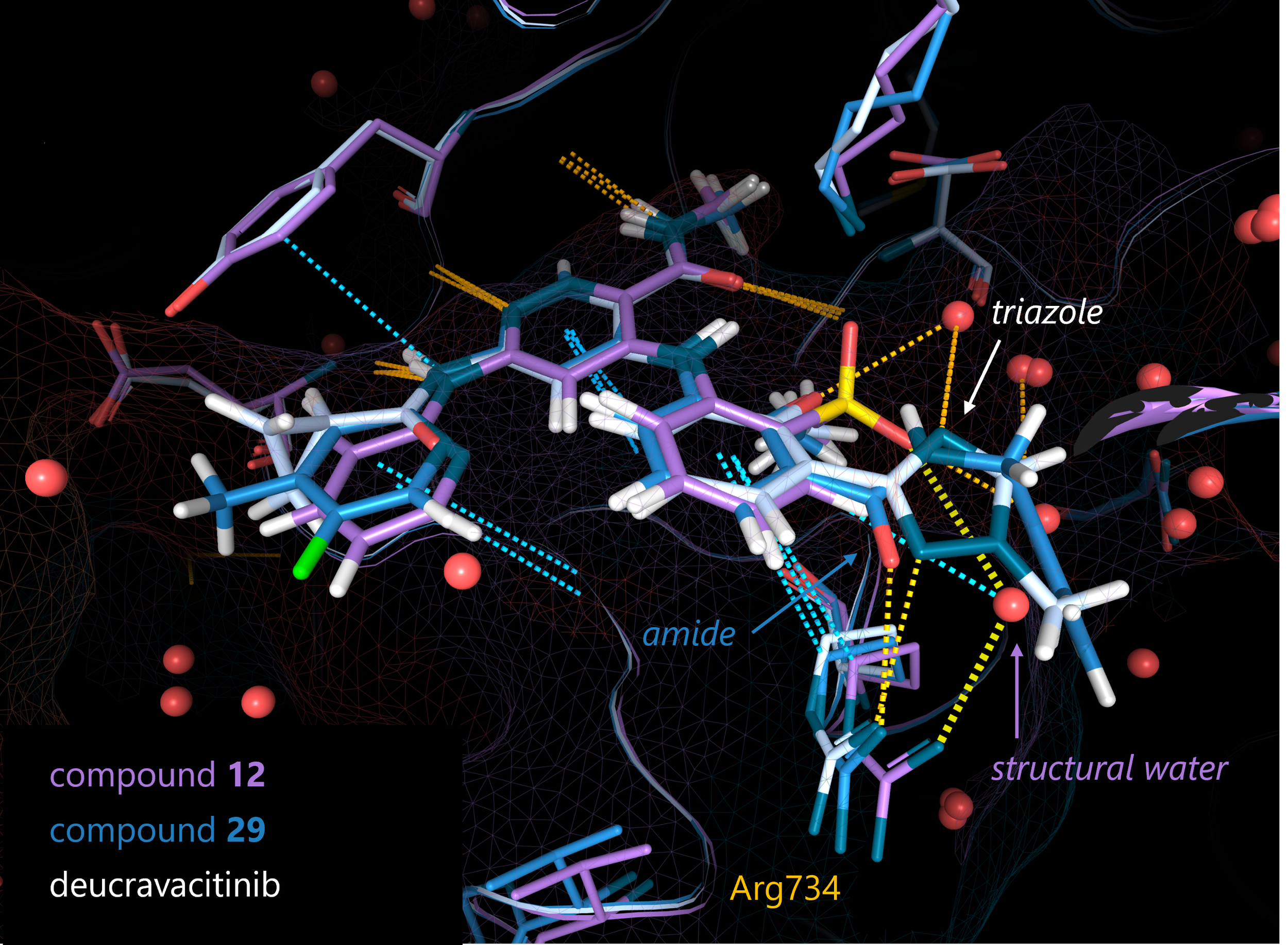
With this objective, they tested several substituents at the C3’ position that could directly form a hydrogen bond with the Arg738 side chain. Compound 29 confirmed their hypothesis: the C3’ amide forms a direct interaction with the desired residue. Moreover, the methoxy group at C2’ helps the binding by forming an intramolecular H-bond with the N-H of the amide in C3’, which stabilizes the amide in the correct conformation for the binding of Arg738.
However, compound 29 had some issues with metabolic stability and permeability, therefore the researchers replaced the C3’ amide with another chemical moiety (a triazole) that still could preserve the interaction with the Arg738. Finally, further modifications to suppress off-target activities provided the drug candidate deucravacitinib. The crystal structure confirmed the success of the water displacement strategy: the designed drug was still able to displace the structural water and directly bind the Arg738.
Image 2. Comparison of the 3D structures of TYK2-JH2 in complex with compound 12 (purple; PDB: 6NZR), compound 29 (blue; PDB: 6NZQ) and deucravacitinib (white; PDB: 6NZP). The original structural water and the optimized substituent are indicated. Picture is produced with the superposition feature and highlight mode of the 3decision® software.
This study confirmed once again how structural insights, in this case about the position of structural water molecules, can be extremely valuable to effectively direct optimization efforts and produce successful drug candidates.
Reference:
Wrobleski ST, Moslin R, Lin S, Zhang Y, Spergel S, Kempson J, Tokarski JS, Strnad J, Zupa-Fernandez A, Cheng L, Shuster D, Gillooly K, Yang X, Heimrich E, McIntyre KW, Chaudhry C, Khan J, Ruzanov M, Tredup J, Mulligan D, Xie D, Sun H, Huang C, D'Arienzo C, Aranibar N, Chiney M, Chimalakonda A, Pitts WJ, Lombardo L, Carter PH, Burke JR, Weinstein DS. Highly Selective Inhibition of Tyrosine Kinase 2 (TYK2) for the Treatment of Autoimmune Diseases: Discovery of the Allosteric Inhibitor BMS-986165. J Med Chem. 2019 Oct 24;62(20):8973-8995. doi: 10.1021/acs.jmedchem.9b00444. Epub 2019 Jul 18. PMID: 31318208.