RAS
June 2021
Doris Alexandra Schuetz, PhD, research advisor
Institute for Research in Immunology and Cancer (IRIC), Université de Montréal, Montreal, Canada
Department of Medicinal Chemistry
The RAS protein belongs to the protein superfamily of small GTPases. RAS is part of the MAP-Kinase signaling pathway and interacts with plenty of proteins to delivers messages in order to control cell growth and malignant transformations. It has been reported that 20% of all tumors show RAS activation by a point mutation in one of the RAS genes. RAS, therefore, presents a prominent cancer target.
RAS (KRAS, NRAS and HRAS), which is the most frequently mutated gene family in cancers, has proven to be a challenging binding interface, as the surface does not provide deep grooves or obvious hydrophobic pockets for small molecules to bind effectively. Research efforts to find an effective RAS inhibitor have been going on for over three decades and it has even been referred to as “undruggable”.
Displacing the nucleotide, which possesses an affinity in the picomolar range to RAS’ orthosteric binding pocket, has not been accomplished in a non-covalent way. Allosteric binding modes of small molecules to RAS have been proposed and crystallographic data has been published. Due to the induced fit mechanism RAS can undergo when interacting with other proteins, I find it most intriguing to target those protein-protein interfaces in order to interfere in the signaling pathway.
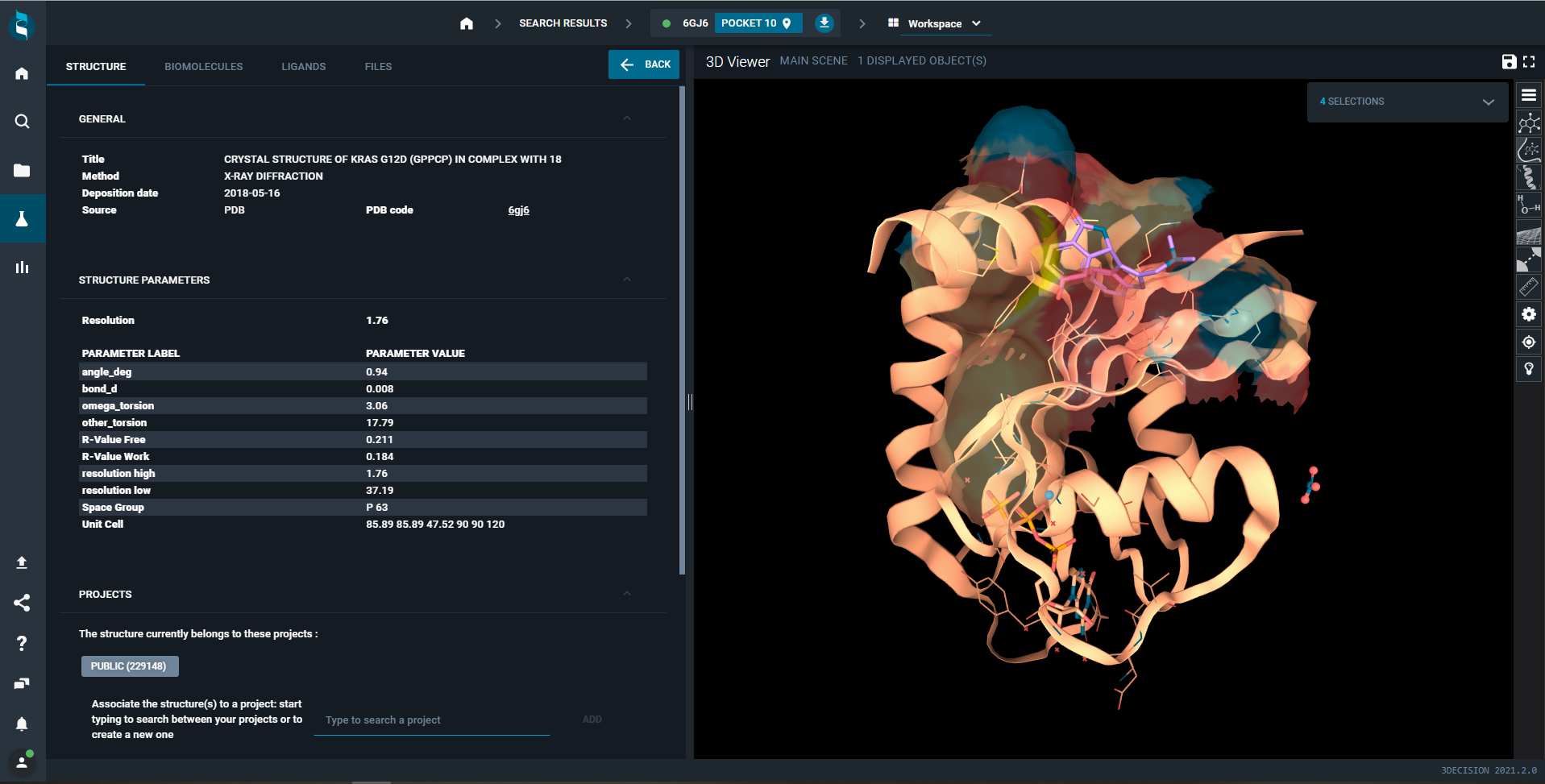
The Ras protein. PDB ID: 6jg6
Targeting Ras signaling to its effectors (GEFs) through small molecules to modulate or disrupt protein-protein interaction is challenging, as the proteins often lack well-defined pockets. Protein-protein interactions typically involve flat and rather large binding sites, which often leads to small molecules displaying low affinity or specificity. Protein-protein interactions, however, remain interesting opportunities for therapeutic intervention. Finding and targeting “hot spots” on the contact surfaces of the proteins employing small molecules can lead to inhibitors showing drug-like potencies. Those small molecules have been shown to bind deeper on the contact surface of the protein than their natural protein partner does.
Thank you Doris for your contribution to our Protein of the Month Initiative!
References: Y. Pylayeva-Gupta, E. Grabocka and D. Bar-Sagi (2011) Ras oncogenes: weaving a tumorigenic web. Nature Reviews Cancer 11, 761-774; I. R. Vetter and A. Wittinghofer (2001) The guanine nucleotide-binding switch in three dimensions. Science 294, 1299-1304; Moore, A.R., Rosenberg, S.C., McCormick, F. et al. RAS-targeted therapies: is the undruggable drugged? Nat Rev Drug Discov 19, 533–552 (2020); Downward, J. Targeting RAS signaling pathways in cancer therapy. Nat Rev Cancer 3, 11–22 (2003)